HIGH DEMAND APPLICATIONS
HIGH PERFORMANCE SANITARY PROTECTION
 Since 1965, Continental Disc Corporation has consistently led the industry in the design and manufacture of innovative, high-quality bursting discs (rupture discs) devices employed in a range of process industries worldwide. These applications include chemical, petrochemical, and petroleum refining, pharmaceutical, beverage, food and dairy, aerospace and electronics industries. CDC maintains an ongoing commitment to providing the products, services, and quality, which customers have come to expect from the leader in over-pressure safety devices. An autoclave is another example of a crucial tool that greatly benefits from the high quality, and durable bursting discs (rupture discs) engineered and manufactured by CDC.
Since 1965, Continental Disc Corporation has consistently led the industry in the design and manufacture of innovative, high-quality bursting discs (rupture discs) devices employed in a range of process industries worldwide. These applications include chemical, petrochemical, and petroleum refining, pharmaceutical, beverage, food and dairy, aerospace and electronics industries. CDC maintains an ongoing commitment to providing the products, services, and quality, which customers have come to expect from the leader in over-pressure safety devices. An autoclave is another example of a crucial tool that greatly benefits from the high quality, and durable bursting discs (rupture discs) engineered and manufactured by CDC.
Autoclaves are steam sterilising chambers designed to maintain a sterile atmosphere, contain and withstand extreme conditions of positive pressure, vacuum, heat and moisture. They play an essential role in a wide range of laboratory and industrial applications, specifically the sterilisation of surgical tools, medical equipment and process media.
Consequently, when autoclaves fail to function properly, the resulting down time leads to extremely expensive and time-consuming inefficiencies. Proper mechanical upkeep of autoclave technologies thus represents a major necessity in developing and maintaining efficient, cost-effective operations. A common source of autoclave malfunction is bursting disc (rupture disc) leakage or premature burst due to fatigue. CDC offers a solution to this problem with the SANITRX HPX bursting disc (rupture disc).
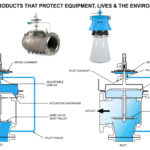
Most autoclaves require bursting disc (rupture disc)s specified to protect the sanitary chamber against system over-pressure. Bursting disc (rupture disc)s not specifically designed to withstand the frequent pressure to vacuum cycles required in these demanding applications can leak or burst prematurely. CDC’s SANITRX HPX Bursting disc (rupture disc) have proven to be durable enough to meet this critical industry need.

CDC’s technical team recently created a solution for a process engineer at a biotechnology facility who was experiencing costly shutdowns. These shutdowns were found to be due to leakage in the bursting discs (rupture discs) used in the autoclave sterilisation equipment at the facility. The CDC team determined that the root cause of this failure was the facility’s utilisation of bursting disc (rupture disc) technology that was developed over 20 years ago and thus no longer the leading technology for the extreme cycle conditions of the autoclave. The result was a fatigued bursting disc (rupture disc) that failed prematurely. CDC solved the problem by replacing all outdated bursting discs (rupture discs) with SANITRX HPX. Built on years of research and development, the HPX Bursting disc (rupture disc) Product Family has proven to withstand over 5,000,000 cycles from full vacuum to 95% of the marked burst rating. Performance at this level represents greater than a five-fold increase over previous industry standards. This extreme durability makes the SANITRX HPX the perfect solution for autoclave pressure protection.
CDC engineers, manufactures and tests each lot of SANITRX HPX bursting discs (rupture discs) to match the precise specifications required to provide the essential over-pressure protection for the equipment and withstand the operating conditions inherent in normal day-to-day autoclave operations. SANITRX HPX bursting discs (rupture disc) incorporate the latest technology features to optimise material thickness, score depth and dome configuration to insure the high performance, flow capacity and durability demanded by today’s process industries. In addition, all SANITRX HPX bursting discs (rupture discs) are designed and manufactured to meet critical ASME BPE criteria including dimensional characteristics required for use in industry standard tubing connections, SF1 or better surface finish, full trace-ability of all materials of construction and FDA/USP Class VI gasket material compliance.
For monitoring burst discs due to an over pressure event, CDC’s BDI or BDI-Flex burst disc indicators are frequently used as part of the SANITRX HPX bursting discs (rupture discs) to automate activation of the over pressure alarm functions in the autoclave control system.
Assentech is proud to supply the Continental Disc Corporation product range. You can contact us by
- Phone 01726 844707 .
- Email : info@assentech.co.uk
- or using the enquiry form below.